- Antibiotics Tests in Milk
- Inhibitory detection test
- Laboratory equipment
- Centrifuges
- Indicator strips
- Autoclaves
- Scales
- Thermometers
- Packing
- PureTrust ATP monitoring
- ATP monitoring PIONEERPRODUKT CleanTrust
- MICROFAST® substrates
- Nutrient media
- Ice cream sticks
- Consumables
- Detergents and disinfectants
- Treatment agent
- Milk filters
- Wipes
- Gloves
- Sampling
Rosselkhoznadzor informs about new rules for regulating the circulation of veterinary medicines for the EAEU countries
The document, approved by the Council of the Eurasian Economic Commission on January 21, 2022, establishes uniform requirements:
— to regulate the circulation of medicines for veterinary use;
— to the procedure for registering these drugs and other procedures related to registration;
— to procedures for assessing the quality, safety and effectiveness of veterinary products and criteria for their assessment;
— to the format of information interaction in the implementation of state control in the field of circulation of veterinary drugs;
— to registers and information databases of the EAEU in the field of circulation of veterinary medicines.
In addition, a ban on the use of a number of drugs for food-producing animals is being introduced in the customs territory of the Eurasian Economic Union.
The presence of a certificate of compliance of the production of veterinary medicinal products with the requirements of the Good Manufacturing Practice Rules becomes a mandatory condition.
Registration of drugs for which an application was submitted in accordance with the legislation of a member state of the EAEU after March 13, 2024 , will be recognized only in the territory of this country.
Considering that the Rules are introduced two years after the entry into force of the Decision of the Council of the Eurasian Economic Commission, a transition period is provided for bringing registration dossiers registered under the legislation of a member state of the EAEU until December 31, 2027.
Also, participants in the circulation of veterinary medicines have time to bring the labeling of drugs into compliance with the Requirements approved by the Decision of the Council of the Eurasian Economic Commission of November 3, 2016 No. 76 before the expiration date of their registration certificates.
The list of drugs is specified in Appendix No. 4 to the Rules for regulating the circulation of veterinary drugs in the customs territory of the Eurasian Economic Union. PIONEER MEIZHENG BIO-TECH (5 in1) JC1165 / Rapid tests for the determination of the residual amount of halofuginone, flavomycin, novobiocin, flunixin, dexamethasone / prednisolone in milk, whey
PIONEER MEIZHENG BIO-TECH (5 in1) JC1165 / Rapid tests for the determination of the residual amount of halofuginone, flavomycin, novobiocin, flunixin, dexamethasone / prednisolone in milk, whey ANTIBIOTICS / ELISA TESTS
ANTIBIOTICS / ELISA TESTS Rapid tests for determining the residual amount of tetracyclines in meat
Rapid tests for determining the residual amount of tetracyclines in meat Express-tests PIONER 5 in1 for the determination of thiamphenicol, meloxicam, colistine, trimethoprim, sulfonamides
Express-tests PIONER 5 in1 for the determination of thiamphenicol, meloxicam, colistine, trimethoprim, sulfonamides Express tests for determining the residual amount of β-lactams and tetracyclines in milk, whey
Express tests for determining the residual amount of β-lactams and tetracyclines in milk, whey Rapid tests PIONER 5 in 1 for the determination of sulfonamides, tylosin, tilmicosin, lincomycin, erythromycin, fluoroquinolones
Rapid tests PIONER 5 in 1 for the determination of sulfonamides, tylosin, tilmicosin, lincomycin, erythromycin, fluoroquinolones- PIONEER MEIZHENG BIO-TECH (5 in1) JC0586 - Antibiotic tests 5 in 1 / Rapid tests for determining the residual amount of β-lactams, tetracyclines and cephalexin in milk, whey
- Express tests for determining the residual amount of β-lactams, tetracyclines, chloramphenicol, streptomycins in milk, whey
- TEST KIT for determination of inhibitory agents PIONEERPRODUKT® DASH-TEST, WC0040
- PIONEER MEIZHENG BIO-TECH (5 in1) JC0871/ Rapid tests for the determination of the residual amount of β-lactams, tetracyclines, chloramphenicol, streptomycins, ceftiofur in milk, whey.
 Stopwatch mechanical stopwatch SOPpr
Stopwatch mechanical stopwatch SOPpr "Keltran" (keltrun) - a complex for the determination of nitrogen and protein by the Kjeldahl method
"Keltran" (keltrun) - a complex for the determination of nitrogen and protein by the Kjeldahl method testo 206-pH2 - Pocket pH meter
testo 206-pH2 - Pocket pH meter Staff table BA-CL- X.X SLP TR - a
Staff table BA-CL- X.X SLP TR - a Milk analyzer Expert Super Breeding
Milk analyzer Expert Super Breeding Tagler TSLM 1-8 milk centrifuge (without heating) (Russia)
Tagler TSLM 1-8 milk centrifuge (without heating) (Russia)- GP dry-air sterilizer (SKTB, Smolensk)
- Milk quality analyzer "Laktan 1-4M" isp. Mini
- Integral C-01 electronic stopwatch
- LOIP LAB-FH-Euro flask heater
- Lumitester SMART Luminometer
- MB25/MB27 moisture content analyzers (Ohaus)
- Smooth micrometers MK
- Mastic Milk Indicator Strips, 100 pcs
- Hydrometers for milk AM, AMT
 Paper for micro-ribbed
Paper for micro-ribbed Parchment
Parchment Laminating paper KH PACK®
Laminating paper KH PACK® Paper sacks
Paper sacks KH PACK® bag making paper
KH PACK® bag making paper Plastic packaging for cakes and pastries
Plastic packaging for cakes and pastries- Siliconized paper for hygiene products
- KH PACK® tartlet paper
- Korreks for desserts
- Salad dressings
- Ice cream chopsticks
- The paper packing fastened anticorrosive UNIK 14-70 THAT 5453-003-05773103-2005
- KH PACK® Straight Packing Paper
- Cover
- Grease and barrier paper KH PACK®
 Ice cream sticks (with logo)
Ice cream sticks (with logo) Ice cream sticks Magnum (curly)
Ice cream sticks Magnum (curly) Petri dish 90 mm
Petri dish 90 mm J-Bottom technology
J-Bottom technology Auxiliaries for sugar products
Auxiliaries for sugar products Ice cream sticks Standard 114
Ice cream sticks Standard 114- GableTop aseptic packaging
- Pepsin whey pork
- General purpose environment of SPC "Biocompass-S" (Uglich)
- Wafer cup and cone
- Ice cream sticks (round)
- Ice cream sticks Standard 93
 Pencils for marking animals
Pencils for marking animals Foaming cup for udder treatment
Foaming cup for udder treatment Fall with a loop for cattle
Fall with a loop for cattle Electric Cattle Driver
Electric Cattle Driver Rubber rings for castration
Rubber rings for castration Mug for milking the first streams of milk.
Mug for milking the first streams of milk.- Disposable udder wipe (udder paper).
- Pre-milking udder cleaner (20 l)
- Forged pitchforks
- Dosing syringe, hose attachment
- Mug for milking the first streams of milk, lengthwise
- Dosing syringe, bottle attachment
- Alkaline detergent (20l / 24kg)
- Plastic syringe 150ml
- Milk filter for fine purification of milk for 2, 5, 10 tons
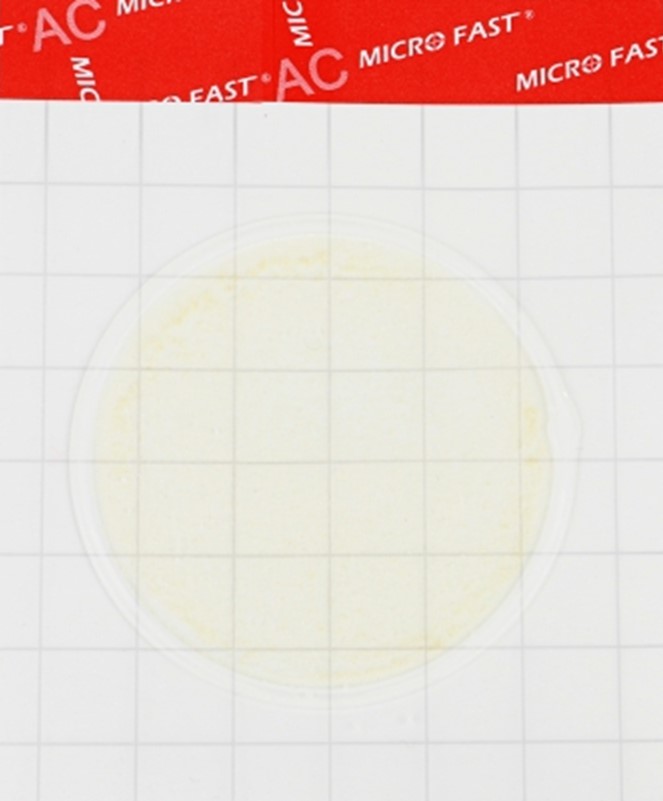 Substrate for determining QMAFAnM (catalog number LR1001)
Substrate for determining QMAFAnM (catalog number LR1001) MicroFast® Staphyloccocus aureus Confirmation Plate Staph.aureus Confirmation Plate (cat. no. LR1005Q)
MicroFast® Staphyloccocus aureus Confirmation Plate Staph.aureus Confirmation Plate (cat. no. LR1005Q) Coliform Count Plate (catalog number LR1002) MicroFast® Coliform Count Plate
Coliform Count Plate (catalog number LR1002) MicroFast® Coliform Count Plate MicroFast® Bacillus cereus Count Plate (catalog number LR1010)
MicroFast® Bacillus cereus Count Plate (catalog number LR1010) MicroFast® Enterobacteriaceae Count Plate (cat. no. LR1011)
MicroFast® Enterobacteriaceae Count Plate (cat. no. LR1011) MicroFast® Microbiological Substrates
MicroFast® Microbiological Substrates- MicroFast® Lactic Acid Bacteria Count Plate (Part Number LR1312)
- MicroFast® Environmental Listeria Count Plate
- MicroFast® Salmonella Count Plate (SAL), for the determination of Salmonella in food and environmental samples (Catalog #LR1006)
- Substrate for accelerated determination of QMAFAnM, (catalog number LR1321)
- MicroFast® Coliform & E.coli Count Plate
- Yeast & Mold Count Plate (cat. no. LR1003) MicroFast® Yeast & Mold Count Plate
- Substrate for determining the number of staphylococci (Catalog number LR1005) MicroFast® Staphyloccocus aureus Count Plate
 Бригадир КСУП "Племзавод "Россь" Волковысского района: мы все работаем на большой урожай21.09.2025
Бригадир КСУП "Племзавод "Россь" Волковысского района: мы все работаем на большой урожай21.09.2025 Почти 40% биржевого экспорта белорусской сельхозпродукции в Россию пришлось на долю Московской области 20.09.2025
Почти 40% биржевого экспорта белорусской сельхозпродукции в Россию пришлось на долю Московской области 20.09.2025 БУТБ будет сотрудничать с торгово-промышленной палатой Камчатского края 19.09.2025
БУТБ будет сотрудничать с торгово-промышленной палатой Камчатского края 19.09.2025- Беларусь и Владимирская область проработают новые направления сотрудничества в биржевой сфере18.09.2025
- The advanced farm of JSC "Gorodilovo" uses the work of scientists and rewards with a trip to the sea18.09.2025
- Беларусь и Египет договорились об активизации диалога в биржевой сфере 17.09.2025
- Минсельхозпрод изменил минимальные экспортные цены на отдельные товары17.09.2025
- Поставки продукции и совместные проекты. Гомельская область и провинция Цзилинь развивают партнерство 17.09.2025
- Промкооперация, АПК, медицина. Определены ключевые направления работы Гродненской и Пензенской областей 17.09.2025
- Сколько сельхозпродукции произвели в Беларуси за январь-август этого года, рассказали в Белстате 17.09.2025
- С работников АПК в Могилевском районе необоснованно удерживали деньги, вмешалась прокуратура17.09.2025
- Belarusian food exports increased by 13% in the first half of the year13.09.2025
- Prizes for the 206th round of the game "Good Luck in Addition!" have been awarded.12.09.2025
- From unique treats to a wish tree. What surprised at the Cheese Festival of the "Molochny Mir" in Grodno12.09.2025
- A modern dairy farm for 777 heads of dairy cattle is being built in the Svisloch district11.09.2025
- Валовое производство молока в Беларуси выросло на 6,5%11.09.2025
 Россия зарегистрировала новый штамм вируса, вызывающего диарею у крупного рогатого скота19.09.2025
Россия зарегистрировала новый штамм вируса, вызывающего диарею у крупного рогатого скота19.09.2025 Россия и Китай нацелились на расширение торговли агропродовольственной продукцией19.09.2025
Россия и Китай нацелились на расширение торговли агропродовольственной продукцией19.09.2025 Новый дрон для мониторинга скота: инновации в агросекторе от МАИ19.09.2025
Новый дрон для мониторинга скота: инновации в агросекторе от МАИ19.09.2025- Меры по предотвращению распространения эпизоотий обсудили на заседании вице-премьера РФ19.09.2025
- Группа «ПРОДО» запускает программу кросс-функционального обучения для работников птицефабрик19.09.2025
- Бывший управляющий птицефабрики должен вернуть 4,8 млн рублей из-за неэффективного управления19.09.2025
- The Ministry of Economic Development is recording deflation in the Russian food market amid rising global prices.19.09.2025
- В Краснооктябрьском сельском поселении введены ограничения из-за бруцеллеза19.09.2025
- Рост цен на говядину в России продолжится: эксперты прогнозируют дальнейшее подорожание19.09.2025
- Производство хамона в Ленобласти расширится на 1,3 миллиарда рублей19.09.2025
- Челябинский фермер погасил долг по ДТП продукцией собственного хозяйства19.09.2025
- Качество куриного мяса в Нижегородской области вызывает опасения: выявлены сальмонеллы и листерии19.09.2025
- Новый порядок GMP-инспекции для производителей ветеринарных препаратов вступит в силу в 2026 году19.09.2025
- Фермер из Коми удвоил производство мяса благодаря государственному гранту18.09.2025
- Предпринимателя в Кирове оштрафовали за продажу шаурмы с сомнительным мясом18.09.2025
- Ставропольский край нацелен на увеличение экспорта сельскохозяйственной продукции в Африку18.09.2025
 10 reasons to take a deposit04.05.2025
10 reasons to take a deposit04.05.2025 Зеленский ввел санкции против внука де Голля, Мизулиной и Гуцул21.09.2025
Зеленский ввел санкции против внука де Голля, Мизулиной и Гуцул21.09.2025 The European Commission has proposed lowering the price ceiling for Russian oil to $47.6 per barrel.20.09.2025
The European Commission has proposed lowering the price ceiling for Russian oil to $47.6 per barrel.20.09.2025- Еврокомиссия предложила ограничить доступ России к технологиям ИИ20.09.2025
- В Испании поддержали использование активов России для помощи Украине20.09.2025
- ЕС намерен ввести санкции против платежной системы «Мир»20.09.2025
- Democrats called for tougher sanctions against Russian energy companies20.09.2025
- Reuters узнал предложение ЕК по точной дате запрета российского СПГ19.09.2025
- ЕК сообщила, что «скоро будут подробности» по санкциям против России19.09.2025
- Handelsblatt reveals EU plans to tighten sanctions against Russia18.09.2025
- Путин продлил действие контрсанкций на два года18.09.2025
- В ЕС заподозрили ловушку в условии Трампа о Китае и пошлинах17.09.2025
- The Foreign Ministry explained why Russia does not raise the issue of lifting sanctions with the US17.09.2025
- Роспатент отказал Renault в регистрации измененного товарного знака17.09.2025
- Песков заявил об открытости России к переговорам по Украине17.09.2025
- Bloomberg узнал сроки проработки новых санкций G7 против России17.09.2025
 В Британии предупредили о риске для миллионов из-за супербактерий06.01.2025
В Британии предупредили о риске для миллионов из-за супербактерий06.01.2025 Moscow court sides with Indian company in dispute with Health Ministry26.11.2024
Moscow court sides with Indian company in dispute with Health Ministry26.11.2024 Scientists estimate increase in mortality due to drug-resistant bacteria29.10.2024
Scientists estimate increase in mortality due to drug-resistant bacteria29.10.2024- Antibiotics for livestock and pesticides found in poisoned family's home29.10.2024
- Izvestia reported on the shortage of widely used antibiotics in Russia29.10.2024
- The Ministry of Health called data on the shortage of antibiotics unreliable29.10.2024
- Scientists warn of threat of return to pre-penicillin times29.10.2024
- The Ministry of Health explained how attitudes towards antibiotics changed during the pandemic07.05.2024
- WHO explains the risks of taking antibiotics "just in case"06.05.2024
- Doctors warn of bad practices after government decision on antibiotics25.04.2024
- The Ministry of Health removed antibiotics and hormones from the standard treatment of ARVI25.04.2024
- Antibiotics in oil: myth or reality?06.03.2024
- Antibiotics in sour cream: myth or reality?05.03.2024
- Antibiotics in goat milk: effects, problems and control measures16.02.2024
- The Japanese will stop producing the popular antibiotic vilprafen in Russia23.12.2023
- Antibiotics in Milk21.12.2023
 Antibiotics in pollock25.02.2024
Antibiotics in pollock25.02.2024 Antibiotics in herring: myth or reality?12.02.2024
Antibiotics in herring: myth or reality?12.02.2024 Antibiotics in perch10.02.2024
Antibiotics in perch10.02.2024- Antibiotics in sprat: facts and myths10.02.2024
- Antibiotics in tuna: an important health and environmental issue09.02.2024
- Antibiotics in meat30.01.2024
- Antibiotics in chebureks: myth or reality?29.01.2024
- Antibiotics in cutlets: problem or myth?18.01.2024
- Antibiotics in Chicken: Where Are the Highest Concentrations?17.01.2024
- Antibiotics in carp17.01.2024
- Where Are More Antibiotics in Chicken: Reality and Cautions16.01.2024
- Antibiotics in Salmon: Safety and Product Quality16.01.2024
- Antibiotics in Turkey15.01.2024
- Antibiotics in Sal: Reality and Safety Issues15.01.2024
- Antibiotics in Fried Dumplings: Facts, Risks and How to Stay Safe15.01.2024
- Antibiotics in sausages14.01.2024
 Antibiotics in Coffee: Myths and Reality03.05.2025
Antibiotics in Coffee: Myths and Reality03.05.2025 Forged forks: 10 interesting facts16.05.2024
Forged forks: 10 interesting facts16.05.2024 Swimming pool and weight loss: 10 interesting facts10.03.2024
Swimming pool and weight loss: 10 interesting facts10.03.2024- Tests for antibiotics in milk - 10 interesting facts07.03.2024
- Cleaning the kettle from scale, 10 interesting facts...06.03.2024
- Antibiotics in beer: 10 interesting facts04.03.2024
- Wild boar, how to survive...01.03.2024
- Purulent mastitis, 10 interesting facts27.02.2024
- Lemon and alcohol: 10 interesting facts25.02.2024
- Mint - 10 interesting facts25.02.2024
- Wild boar, 10 interesting facts20.02.2024
- Wild boar and domestic pig: comparison and advantages20.02.2024
- Cottage cheese, 10 interesting facts20.02.2024
- 10 Interesting Facts About Milk19.02.2024
- How to Clean a Toilet - 10 Interesting Facts (Acid vs Alkaline)18.02.2024
- Goat's milk: 10 interesting facts16.02.2024
 Dicroceliosis in cattle09.03.2024
Dicroceliosis in cattle09.03.2024 Demodicosis in cattle01.03.2024
Demodicosis in cattle01.03.2024 Purulent mastitis of cattle27.02.2024
Purulent mastitis of cattle27.02.2024- Hypodermatosis in cattle20.02.2024
- Hemonchoz in cattle11.02.2024
- Bursitis in cattle30.01.2024
- Brucellosis in cattle29.01.2024
- Bronchopneumonia in calves27.01.2024
- Bronchitis in cattle26.01.2024
- Mortellaro disease in cattle24.01.2024
- White muscle disease in cattle23.01.2024
- Babesiosis in cattle22.01.2024
- Cattle acidosis20.01.2024
- Arthritis in cattle20.01.2024
- Anaplasmosis in cattle18.01.2024
 Antibiotics for coughs: when they are needed and when they are not11.02.2024
Antibiotics for coughs: when they are needed and when they are not11.02.2024 Борьба с контрабандой, переориентация грузов. Как работают белорусские таможенники21.09.2025
Борьба с контрабандой, переориентация грузов. Как работают белорусские таможенники21.09.2025 Как будут регулировать интернет-торговлю в Беларуси? МАРТ предложил обсудить проект указа 20.09.2025
Как будут регулировать интернет-торговлю в Беларуси? МАРТ предложил обсудить проект указа 20.09.2025- В Гродно работник СТО взял автомобиль, который сдали для химчистки, и попал в ДТП19.09.2025
- Основная причина пожаров в Могилевской области в этом году - неосторожное обращение с огнем 19.09.2025
- Вели себя неадекватно в зоопарке и снимали на видео: в Минске задержали двоих нарушителей порядка 19.09.2025
- В Гомельской области мужчина выстрелил из пневматики по ногам дочери, возбуждено уголовное дело 19.09.2025
- На Корсике самолет долго не мог приземлиться из-за уснувшего авиадиспетчера18.09.2025
- Прокуратура Верхнедвинского района выявила социально опасное положение ребенка после трансляции в TikTok18.09.2025
- Велосипедист погиб в ДТП с грузовиком в Вилейском районе18.09.2025
- ГАИ Могилевской области за прошедшие выходные оштрафовала 77 пешеходов 17.09.2025
- В Витебской области за выходные задержали 12 нетрезвых водителей17.09.2025
- "Белгоспищепром": наши производители напитков постоянно работают над освоением новых технологий17.09.2025
- "Это даже не проблема - это дисфункция!" Откуда берутся проблемы с эрекцией и что делать?14.09.2025
- Управляемое пьяным водителем авто врезалось в другое на перекрестке в Минске14.09.2025
- Пьяный минчанин-бесправник угнал такси и врезался в жилой дом13.09.2025
Persons
Our Partners
Top 10
Our Test - Pioneer Tests
- Express tests for determining the residual amount of β-lactams, tetracyclines, chloramphenicol, streptomycins in milk, whey
- TEST KIT for determination of inhibitory agents PIONEERPRODUKT® DASH-TEST, WC0040
- PIONEER MEIZHENG BIO-TECH (5 in1) JC0586 - Antibiotic tests 5 in 1 / Rapid tests for determining the residual amount of β-lactams, tetracyclines and cephalexin in milk, whey
- PIONEER MEIZHENG BIO-TECH (5 in1) JC0871/ Rapid tests for the determination of the residual amount of β-lactams, tetracyclines, chloramphenicol, streptomycins, ceftiofur in milk, whey.
- PIONEER MEIZHENG BIO-TECH (5 in1) JC1165 / Rapid tests for the determination of the residual amount of halofuginone, flavomycin, novobiocin, flunixin, dexamethasone / prednisolone in milk, whey

